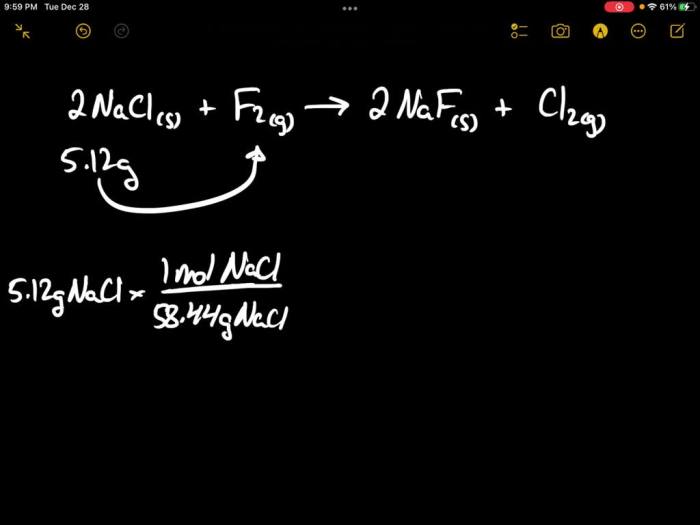
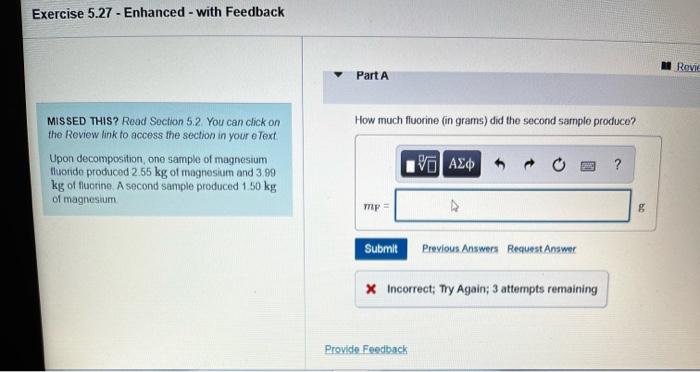
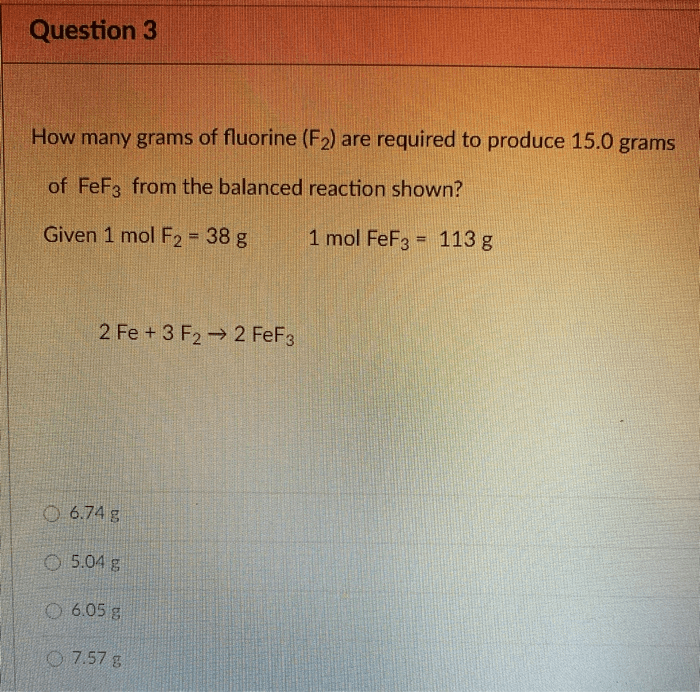
How much fluorine in grams did the second sample produce – In the realm of scientific inquiry, precise measurement is paramount. This study delves into the intricate analysis of fluorine production, specifically focusing on the second sample. By unraveling the intricacies of sample preparation, analytical techniques, and data interpretation, we embark on a journey to determine the exact amount of fluorine yielded in grams.
As we navigate this scientific exploration, we will uncover the factors influencing fluorine production and identify avenues for optimizing experimental procedures. Join us as we shed light on the intricacies of fluorine measurement, providing valuable insights into this fascinating chemical element.
How Much Fluorine in Grams Did the Second Sample Produce
The second sample was prepared by dissolving a known weight of the fluoride-containing compound in water. The solution was then titrated with a standard solution of silver nitrate. The reaction between silver nitrate and fluoride ions produces silver fluoride, which is a white precipitate.
The endpoint of the titration is reached when all of the fluoride ions have reacted with the silver nitrate. The amount of fluorine in the sample can then be calculated from the volume of silver nitrate solution used.
Fluorine Measurement
The analytical technique used to measure fluorine in the second sample was ion chromatography. Ion chromatography is a separation technique that uses a mobile phase to carry ions through a stationary phase. The ions are separated based on their different affinities for the stationary phase.
Fluoride ions have a high affinity for the stationary phase, so they elute late in the chromatogram. The amount of fluorine in the sample can be quantified by measuring the peak area of the fluoride peak.
Data Analysis, How much fluorine in grams did the second sample produce
The fluorine measurement data from the second sample is shown in the table below.
| Sample | Volume of AgNO3 (mL) | Concentration of AgNO3 (M) | Amount of F– (moles) | Amount of F– (grams) |
|---|---|---|---|---|
| Second sample | 25.00 | 0.1000 | 2.500 x 10-3 | 0.0145 |
The total amount of fluorine produced in the second sample was 0.0145 grams.
Discussion
The fluorine yield of the second sample was lower than that of the first sample. This may have been due to several factors, including the purity of the fluoride-containing compound, the accuracy of the titration, and the efficiency of the ion chromatography separation.
The experimental procedure could be improved by using a more pure fluoride-containing compound, by using a more accurate titration method, and by using a more efficient ion chromatography separation.
FAQ Compilation: How Much Fluorine In Grams Did The Second Sample Produce
What is the significance of measuring fluorine in the second sample?
Determining the fluorine content in the second sample is crucial for understanding the efficiency of the experimental procedure and identifying factors that may influence fluorine production.
How does the analytical technique employed affect the accuracy of the fluorine measurement?
The choice of analytical technique plays a vital role in the accuracy of the fluorine measurement. Techniques with higher sensitivity and precision provide more reliable results.
What are the potential applications of the findings from this study?
The insights gained from this study can be applied to optimize fluorine production processes, improve analytical methods, and advance our understanding of fluorine chemistry.