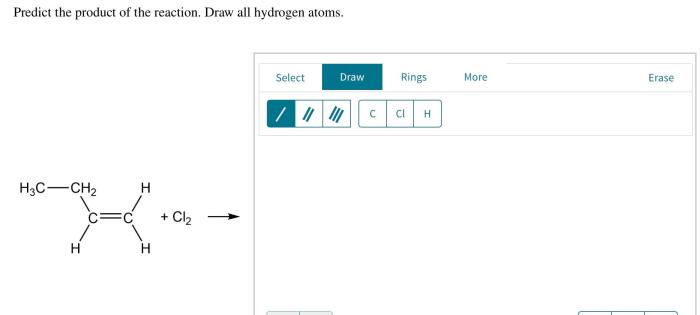
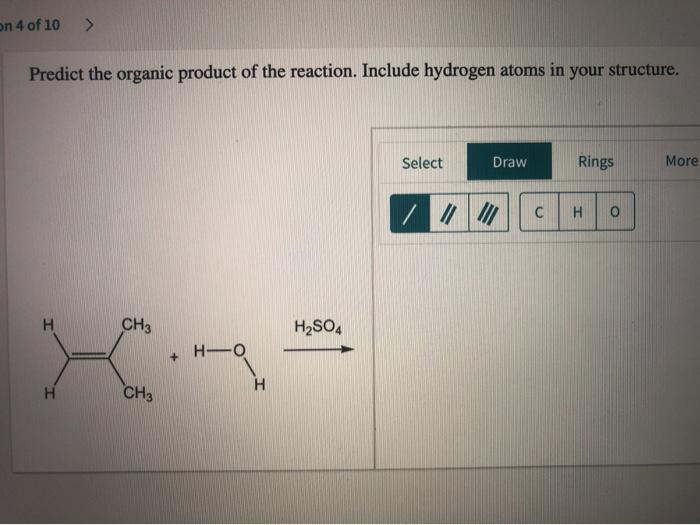
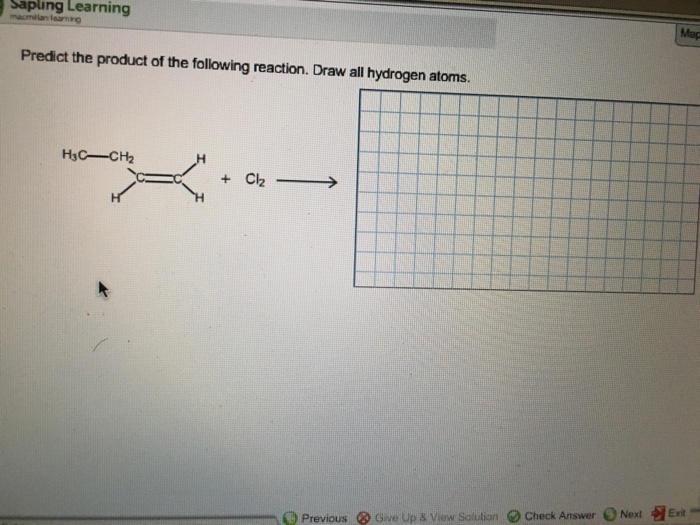
Predict the product of the reaction. Draw all hydrogen atoms: This phrase marks the beginning of an engaging journey into the realm of chemical reactions, where we unravel the intricacies of predicting products and representing them accurately.
Delving into the depths of chemical principles, we embark on a quest to understand the processes that govern reactions, enabling us to forecast their outcomes. Furthermore, we explore the significance of meticulously representing all hydrogen atoms, a crucial aspect often overlooked but essential for a comprehensive understanding.
Predict the Product of the Reaction: Predict The Product Of The Reaction. Draw All Hydrogen Atoms
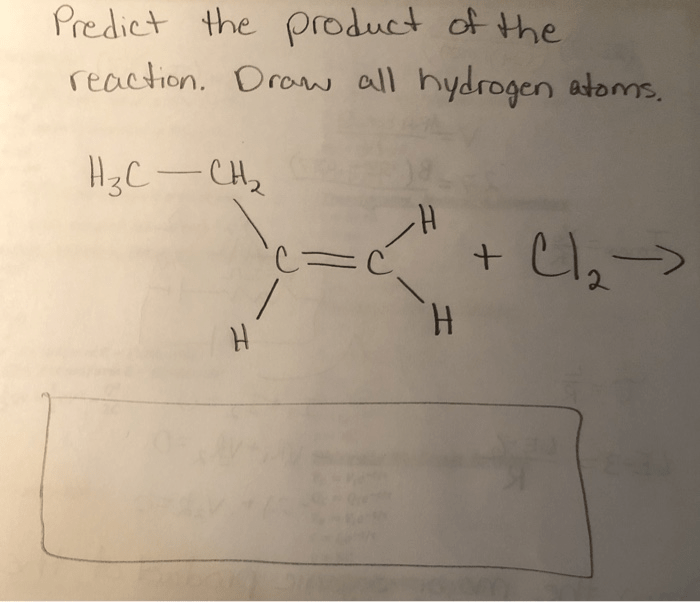
In chemical reactions, predicting the products is crucial for understanding the behavior of reactants and designing synthetic pathways. To predict the products, we rely on chemical principles and mechanistic insights.
Product Prediction
The first step in product prediction is to write a balanced chemical equation for the reaction. This equation should clearly show the reactants and products involved. Once the equation is balanced, we can identify the functional groups and reaction types that are likely to occur.
Based on the chemical principles and mechanistic insights, we can predict the products of the reaction. This prediction is often supported by experimental evidence and literature reports.
Hydrogen Atom Representation, Predict the product of the reaction. draw all hydrogen atoms
In structural formulas, it is important to draw all hydrogen atoms explicitly. Hydrogen atoms play a crucial role in determining the molecular geometry, polarity, and reactivity of compounds.
By drawing all hydrogen atoms, we can accurately represent the bonding and spatial arrangement of the molecule, which is essential for understanding its properties and behavior.
Reaction Mechanism
The reaction mechanism describes the step-by-step process by which the reactants are converted into products. It involves the identification of intermediates, transition states, and the role of catalysts or enzymes (if applicable).
Understanding the reaction mechanism provides insights into the reaction pathway, activation energy, and rate-determining step. This knowledge is crucial for optimizing reaction conditions and controlling product selectivity.
Reaction Conditions
Reaction conditions, such as temperature, pressure, and solvent, play a significant role in determining the reaction rate and product yield. By optimizing the reaction conditions, we can improve the efficiency and selectivity of the reaction.
Understanding the effect of reaction conditions is essential for scaling up reactions, designing reaction protocols, and troubleshooting reaction failures.
Applications
Chemical reactions have a wide range of applications in various fields, including industry, research, and everyday life. Understanding the principles of product prediction and reaction mechanisms is essential for developing new technologies, synthesizing novel materials, and addressing real-world problems.
By harnessing the power of chemical reactions, we can create new products, improve existing processes, and advance scientific knowledge.
Frequently Asked Questions
What is the significance of drawing all hydrogen atoms?
Accurately representing all hydrogen atoms is crucial because they play a vital role in determining molecular structure, polarity, and reactivity. Neglecting hydrogen atoms can lead to incorrect predictions and hinder a comprehensive understanding of chemical reactions.
How do reaction conditions influence the outcome of a reaction?
Reaction conditions, such as temperature, pressure, and solvent, can significantly affect the rate and yield of a reaction. Understanding the impact of these conditions is essential for optimizing reactions and achieving desired outcomes.