Ranking task atomic energy levels and photons – Ranking atomic energy levels and photons is a fundamental task in understanding the behavior of atoms and their interactions with light. This ranking provides insights into the electronic structure of atoms, the transitions between energy levels, and the emission and absorption of photons.
This article delves into the concepts, experimental techniques, and applications of ranking atomic energy levels and photons, exploring their significance in various fields of science.
The ranking of atomic energy levels is influenced by factors such as the nuclear charge, electron-electron interactions, and the angular momentum of electrons. Photons play a crucial role in the transitions between energy levels, carrying energy equal to the difference between the levels involved.
Understanding the ranking of atomic energy levels and the role of photons is essential for advancing our knowledge in atomic physics, spectroscopy, and quantum mechanics.
Ranking Task of Atomic Energy Levels
The ranking of atomic energy levels is a fundamental concept in atomic physics that describes the ordering of energy states within an atom. It plays a crucial role in understanding the behavior of atoms and their interactions with light and other particles.
The ranking of atomic energy levels is influenced by several factors, including the number of electrons in the atom, the nuclear charge, and the electron-electron interactions. The energy levels are typically arranged in a hierarchical structure, with the lowest energy level (ground state) at the bottom and the higher energy levels (excited states) above it.
Examples of Ranking Atomic Energy Levels
- For hydrogen atom, the energy levels are ranked as 1s, 2s, 2p, 3s, 3p, 3d, etc., with 1s being the ground state.
- For helium atom, the energy levels are ranked as 1s 2, 2s 2, 2p 2, 3s 2, 3p 2, 3d 2, etc., with 1s 2being the ground state.
- For sodium atom, the energy levels are ranked as 1s 22s 22p 63s, 1s 22s 22p 63p, 1s 22s 22p 63d, etc., with 1s 22s 22p 63s being the ground state.
Role of Photons in Energy Level Transitions
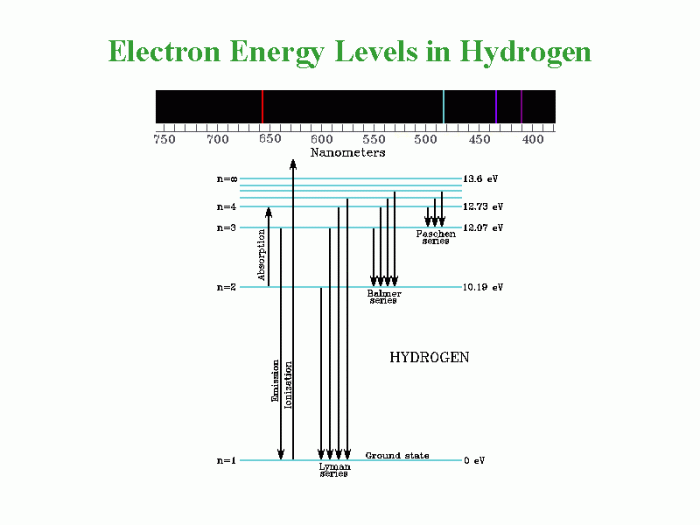
Photons play a crucial role in the transitions between atomic energy levels. When an atom absorbs a photon of energy equal to the energy difference between two energy levels, an electron can move from a lower energy level to a higher energy level.
Conversely, when an electron moves from a higher energy level to a lower energy level, a photon of energy equal to the energy difference between the two levels is emitted.
Examples of Photon Absorption and Emission
- When an atom absorbs a photon of energy equal to the energy difference between the ground state and the first excited state, the electron moves from the ground state to the first excited state.
- When an electron moves from the first excited state to the ground state, a photon of energy equal to the energy difference between the two states is emitted.
- The absorption and emission of photons by atoms are the basis of many spectroscopic techniques used to study atomic structure and dynamics.
Experimental Methods for Determining Atomic Energy Level Rankings: Ranking Task Atomic Energy Levels And Photons
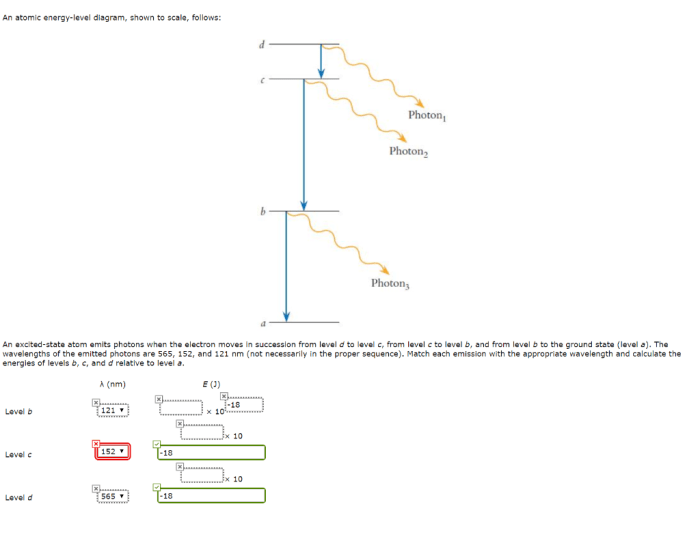
There are several experimental techniques used to determine the ranking of atomic energy levels. One of the most common techniques is spectroscopy.
Spectroscopy
Spectroscopy involves the study of the interaction of light with atoms and molecules. By analyzing the wavelengths of light absorbed or emitted by atoms, it is possible to determine the energy differences between the atomic energy levels.
Other Experimental Methods
- Atomic beam spectroscopy
- Laser-induced fluorescence
- Photoelectron spectroscopy
Applications of Atomic Energy Level Ranking
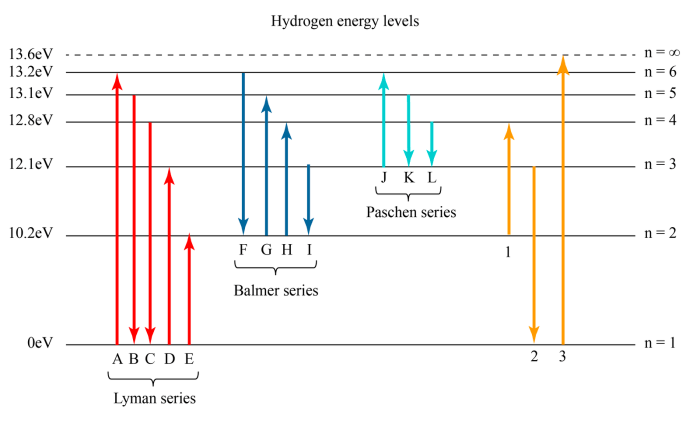
The ranking of atomic energy levels has a wide range of applications in various fields.
Atomic Structure and Spectroscopy
The ranking of atomic energy levels is essential for understanding the structure of atoms and their interactions with light. It is used in the development of spectroscopic techniques for studying atomic properties and dynamics.
Quantum Mechanics
The ranking of atomic energy levels provides experimental evidence for the quantum mechanical model of the atom. It helps to validate the theoretical predictions of quantum mechanics and provides insights into the fundamental principles governing atomic behavior.
Astrophysics
The ranking of atomic energy levels is used in astrophysics to study the composition and properties of stars and other celestial objects. By analyzing the light emitted or absorbed by atoms in stars, astronomers can determine the temperature, density, and chemical composition of these objects.
Laser Physics
The ranking of atomic energy levels is essential for the development and operation of lasers. Lasers rely on the stimulated emission of photons by atoms, and the energy difference between the atomic energy levels determines the wavelength of the laser light.
Chemical Analysis, Ranking task atomic energy levels and photons
The ranking of atomic energy levels is used in chemical analysis to identify and quantify the elements present in a sample. Spectroscopic techniques based on atomic energy level transitions are widely used in analytical chemistry.
Computational Methods for Atomic Energy Level Ranking
In addition to experimental methods, computational methods are also used to calculate and predict the ranking of atomic energy levels.
Hartree-Fock Theory
Hartree-Fock theory is a self-consistent field method used to approximate the wavefunction and energy levels of atoms and molecules. It provides a starting point for more accurate calculations.
Density Functional Theory
Density functional theory is a more advanced computational method that takes into account the electron correlation effects. It is widely used for calculating the energy levels and properties of atoms and molecules.
Accuracy and Limitations
Computational methods can provide accurate predictions of atomic energy level rankings, but their accuracy depends on the level of theory used and the size of the system being studied.
FAQ Overview
What is the significance of ranking atomic energy levels?
Ranking atomic energy levels provides insights into the electronic structure of atoms, allowing us to understand the behavior of electrons and their interactions within the atom.
How do photons contribute to the ranking of atomic energy levels?
Photons are involved in transitions between atomic energy levels, carrying energy equal to the difference between the levels involved. This relationship enables the determination of energy level rankings through the study of photon emission and absorption.
What experimental techniques are used to determine atomic energy level rankings?
Experimental techniques such as spectroscopy, atomic beam spectroscopy, and laser-induced fluorescence are employed to measure the wavelengths of photons emitted or absorbed during atomic energy level transitions, providing data for ranking the energy levels.
What are the practical applications of ranking atomic energy levels?
Ranking atomic energy levels has applications in fields such as astrophysics, laser physics, and chemical analysis. It aids in understanding atomic structure, predicting spectral lines, and developing lasers with specific wavelengths.